FDA May Reclassify ECT Devices
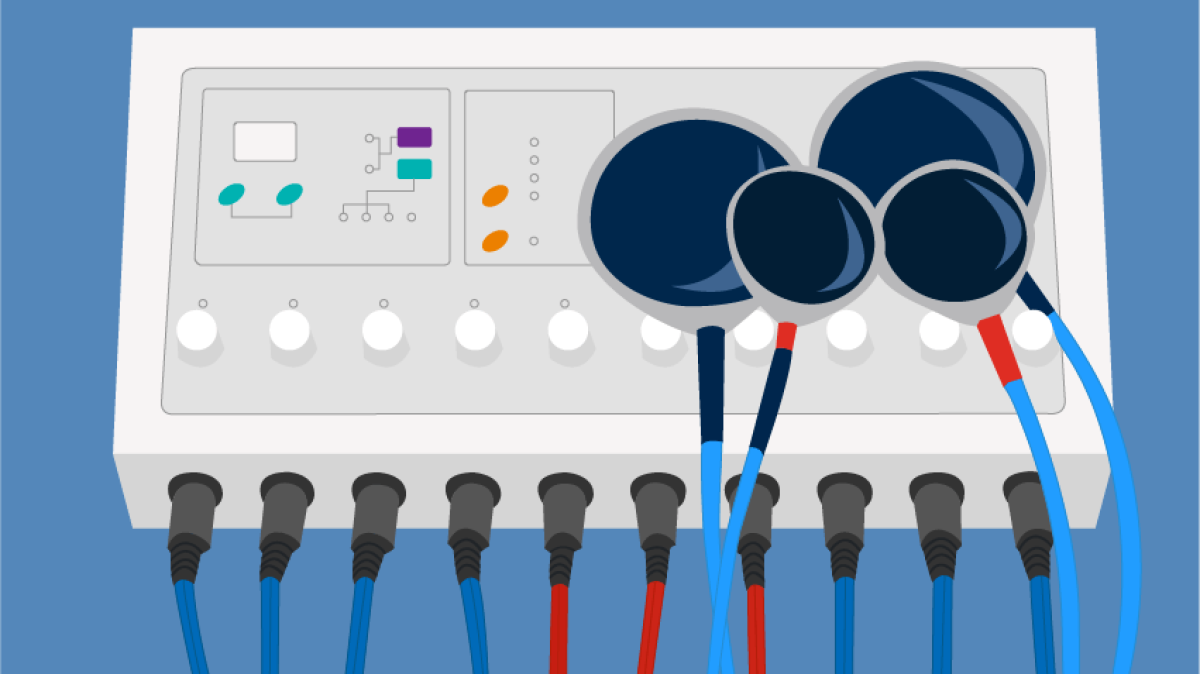
FDA May Reclassify ECT Devices
The FDA may reclassify electroconvulsive therapy devices from class III to class II. The FDA is accepting public comments on the change until March 28.
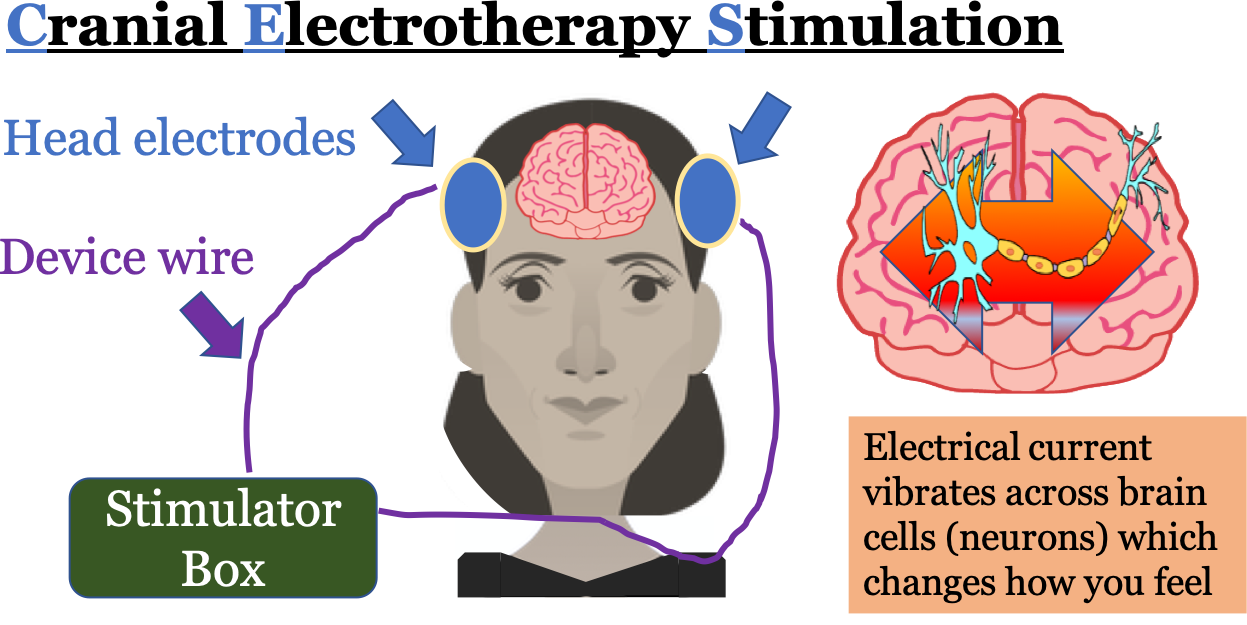
What is Cranial Electrotherapy Stimulation (CES)?

How to Report Your ECT Injury to the FDA - Life After ECT

Life After ECT Podcast on Spotify
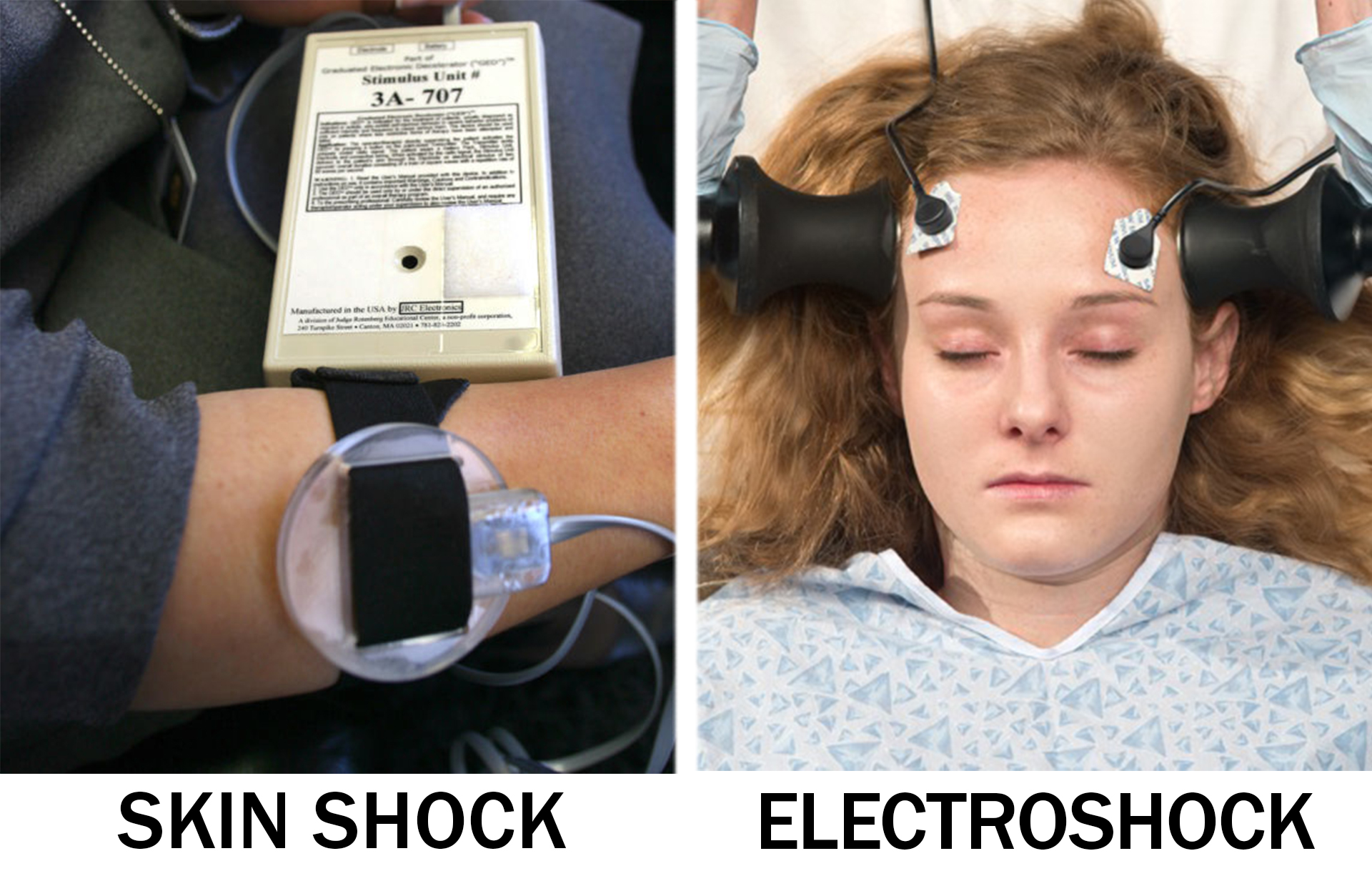
FDA regulates use of two electric shock devices for children's
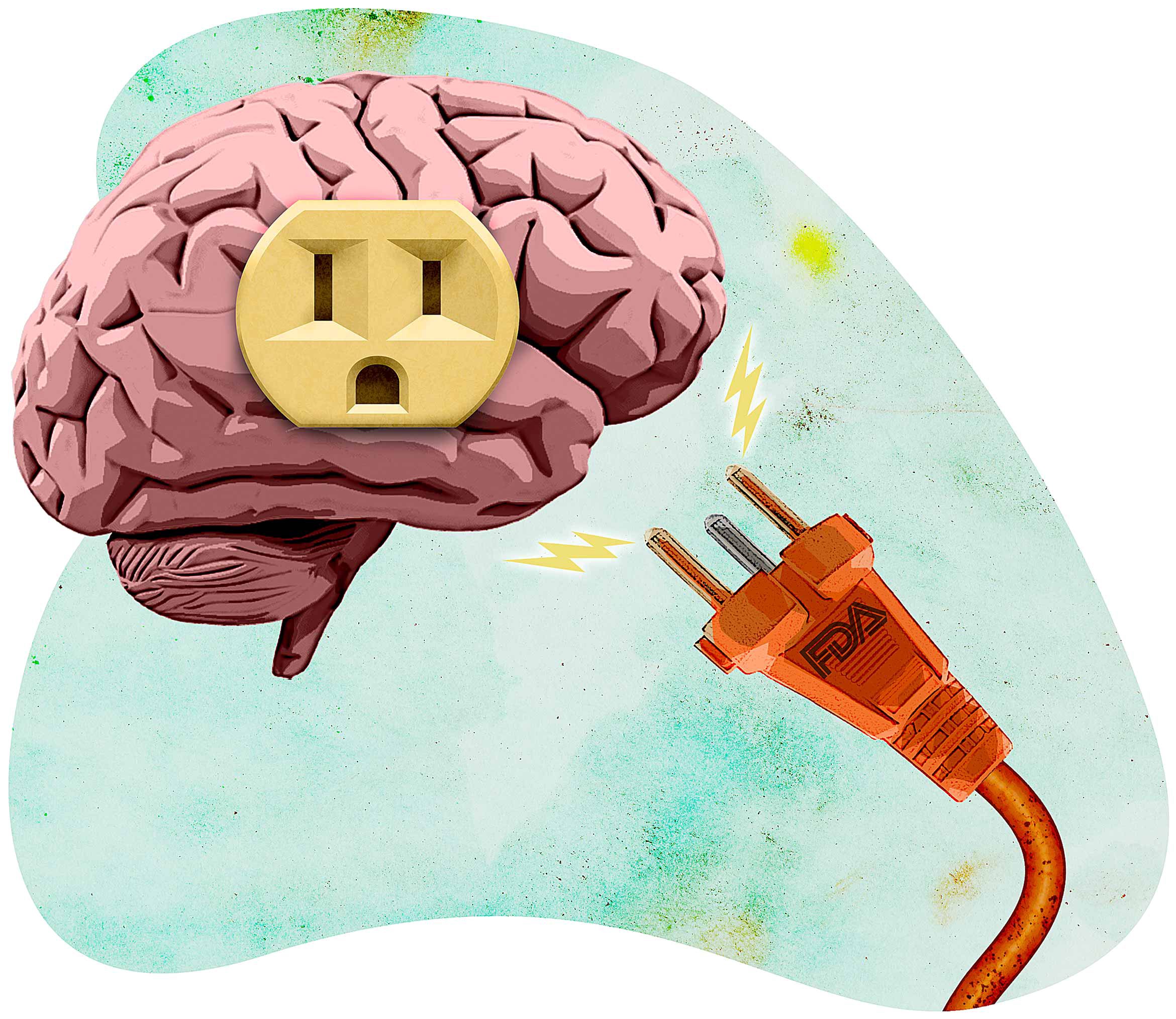
FDA unjustified in downgrading shock therapy brain injury risks
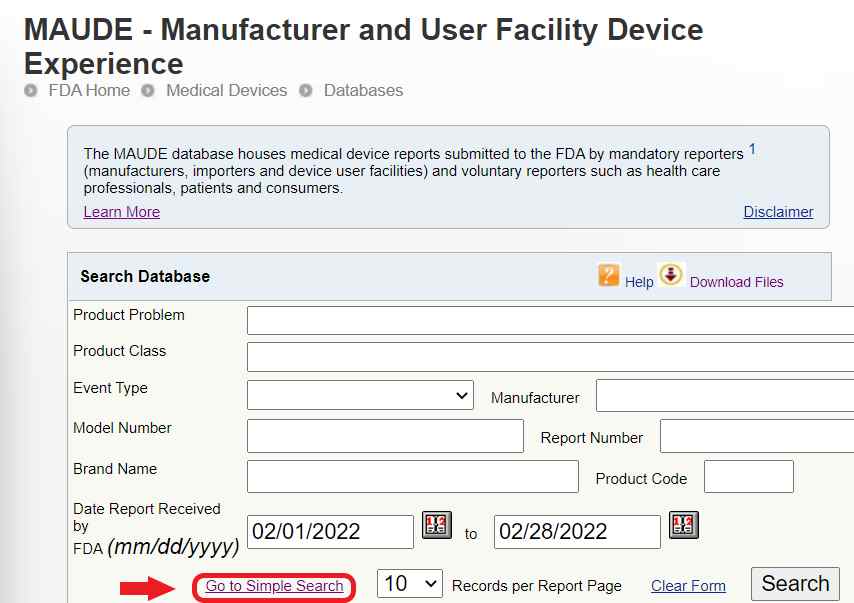
How to Report Your ECT Injury to the FDA - Life After ECT

Advocacy Update: February 2019
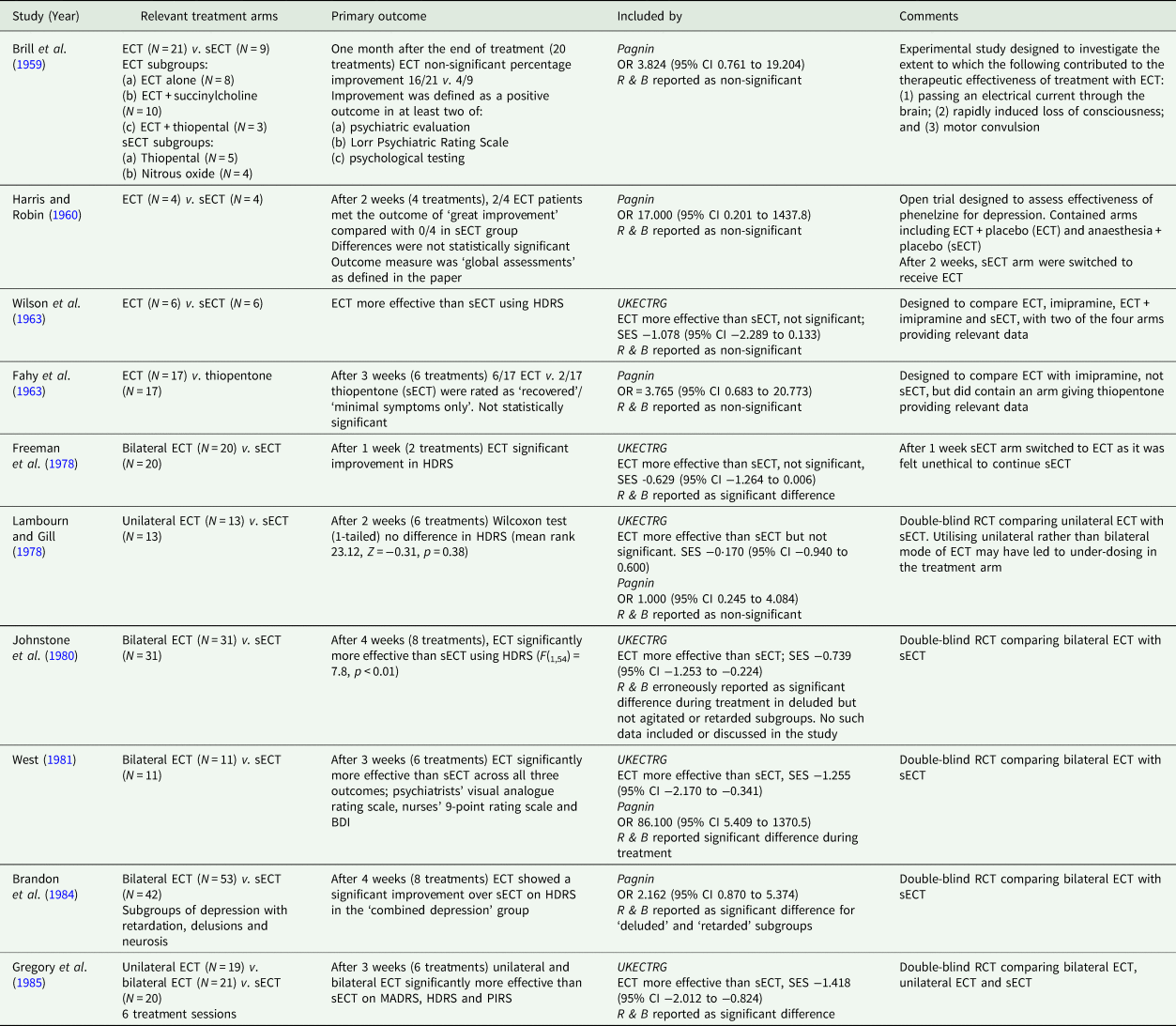
A critique of narrative reviews of the evidence-base for ECT in

10 Facts You May Not Know About Electroconvulsive Therapy (ECT


