Ice - Structure, Formation, Properties

Ice - Structure, Formation, Properties
Ice - Structure, Formation, Properties: At standard atmospheric pressure and at temperatures near 0 °C, the ice crystal commonly takes the form of sheets or planes of oxygen atoms joined in a series of open hexagonal rings. The axis parallel to the hexagonal rings is termed the c-axis and coincides with the optical axis of the crystal structure. When viewed perpendicular to the c-axis, the planes appear slightly dimpled. The planes are stacked in a laminar structure that occasionally deforms by gliding, like a deck of cards. When this gliding deformation occurs, the bonds between the layers break, and the hydrogen atoms involved in those.
Ice, solid substance produced by the freezing of water vapour or liquid water. At temperatures below 0 °C (32 °F), water vapour develops into frost at ground level and snowflakes (each of which consists of a single ice crystal) in clouds. Below the same temperature, liquid water forms a solid, as

Structural glaciology
Foods, Free Full-Text

The smallest ice crystals in the world

How does Arctic sea ice form and decay - Wadhams

Formation of Ice-like Water Structure on the Surface of an Antifreeze Protein
Science of Sea Ice National Snow and Ice Data Center
Structure of Water and Hydrogen Bonding

Why Does Water Expand When It Freezes? » Science ABC

Formation and properties of ice XVI obtained by emptying a type sII clathrate hydrate

Ice - Structure, Formation, Properties
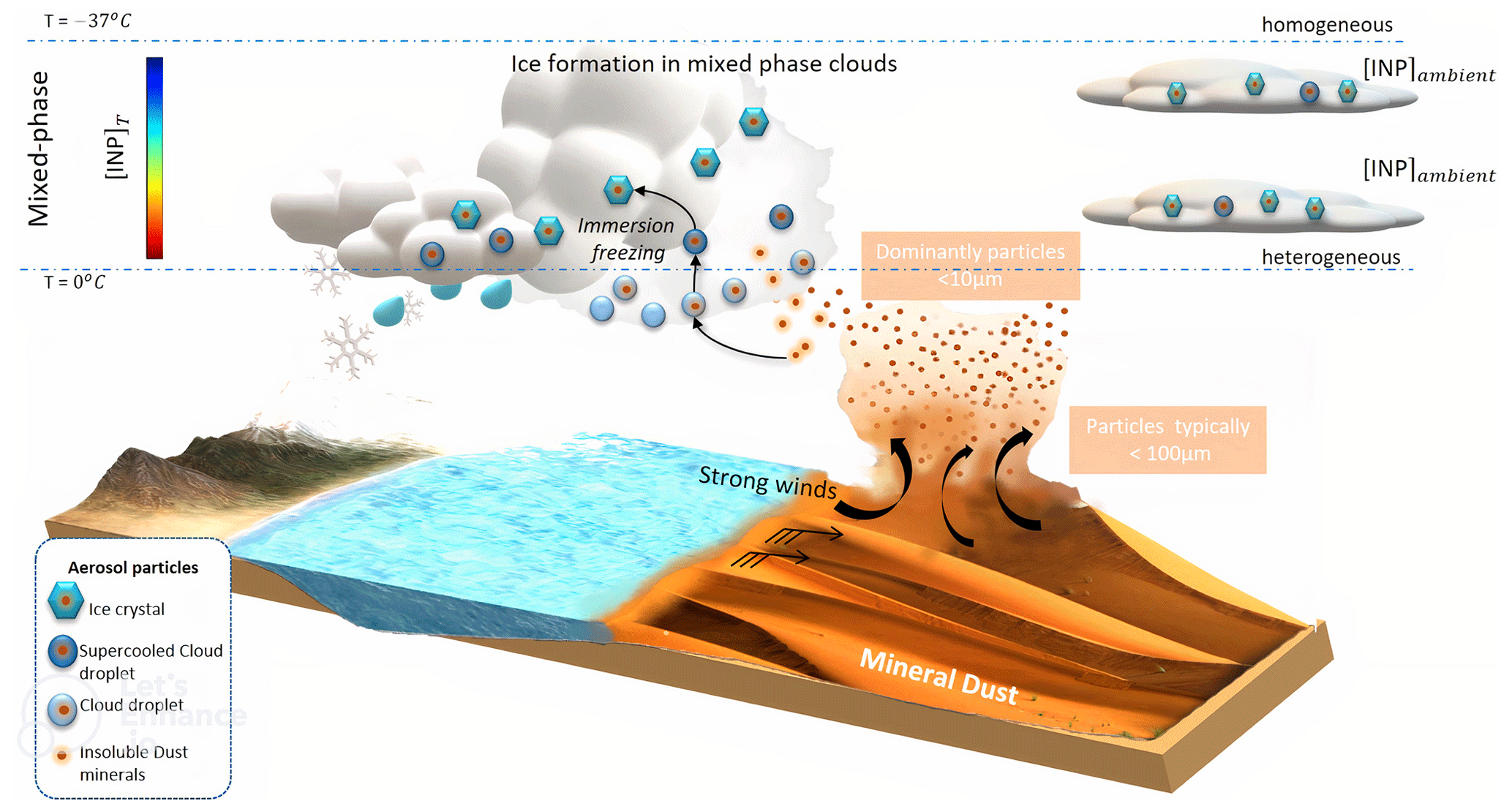
ACP - Role of K-feldspar and quartz in global ice nucleation by mineral dust in mixed-phase clouds


